The emergence of mpox resistant tecovirimat has sparked significant concern among health officials, especially in light of the increasing number of cases linked to a tecovirimat-resistant monkeypox virus (MPXV) in the United States. With the monkeypox outbreak 2024 presenting new challenges, the ability of the virus to resist current treatment options underscores the urgent need for effective solutions. The CDC has issued updates highlighting the evolving landscape of mpox treatment options, stressing that tecovirimat resistance could hinder efforts to control the outbreak. As the situation progresses, drug-resistant monkeypox poses a serious threat to public health, necessitating thorough monitoring and research on alternative therapies. Understanding tecovirimat resistance is crucial, as it may redefine strategies in managing this infectious disease and protect vulnerable populations.
The recent identification of a tecovirimat-resistant strain of the monkeypox virus highlights an alarming trend in the treatment landscape for mpox. This adaptation in the virus signifies the challenges faced by health authorities in managing ongoing outbreaks effectively. Various treatment alternatives are being sought as the current reliance on tecovirimat becomes questionable due to emerging resistance. The situation emphasizes the critical need for a comprehensive approach to combating the monkeypox outbreak in 2024, as well as the importance of genomic surveillance in detecting resistant variants. In light of these developments, discussions around effective management strategies and alternative therapeutics are more pertinent than ever.
Understanding Tecovirimat Resistance in Mpox Cases
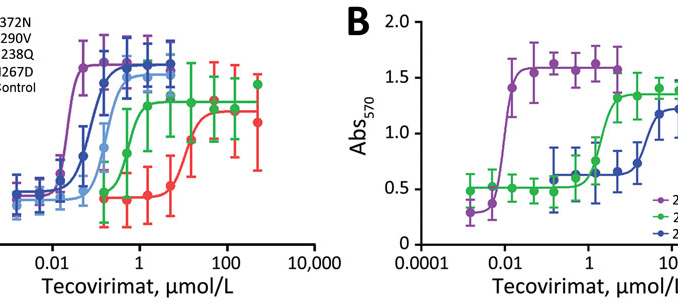
The emergence of tecovirimat-resistant monkeypox viruses (MPXV) has raised significant concerns among health authorities, particularly in the context of a recent cluster of mpox cases in the United States. This cluster involved individuals who had no prior exposure to tecovirimat, highlighting the potential for the virus to develop resistance even in treatment-naïve patients. As of 2024, reports indicate that mutations, specifically F13 mutations, linked to drug resistance have been observed in multiple states, underscoring the urgent need for ongoing surveillance and research into the mechanisms of resistance.
Epidemiologists, including Dr. Crystal M. Gigante from the CDC, have emphasized that while tecovirimat has become a widely utilized treatment option during the ongoing mpox outbreak, its investigational status and lack of FDA approval underscore the importance of further studies. The unexpected emergence of resistant strains raises questions about the long-term effectiveness of tecovirimat and the potential implications for mpox treatment protocols in the future.
Mpox Treatment Options Beyond Tecovirimat
Given the current landscape of mpox treatment options, the rising incidence of tecovirimat-resistant strains necessitates a broader exploration of alternative therapeutics. The CDC has highlighted the crucial need for developing additional treatment modalities to ensure that healthcare providers can effectively manage mpox infections, especially among high-risk populations. The absence of approved first-line treatments could result in detrimental health outcomes for individuals with severe immunocompromising conditions, emphasizing the need for innovative solutions.
Current clinical trials, such as the National Institutes of Health’s STOMP study, aim to evaluate the efficacy of tecovirimat and potentially identify new treatment strategies for mpox. As researchers gather data from these trials, it is essential for healthcare providers to stay informed about the latest findings and consider enrolling patients in ongoing studies. This collaborative approach could pave the way for discovering new therapies that can complement or replace tecovirimat in the management of mpox.
Impact of the 2024 Monkeypox Outbreak
The monkeypox outbreak of 2024 has emerged as a significant public health challenge, particularly with the detection of tecovirimat-resistant MPXV variants. Health officials are closely monitoring the situation, recognizing the need for immediate action to curb the spread of these resistant strains. The identification of F13 mutations among patients across multiple states has prompted enhanced genomic surveillance efforts to track the virus’s evolution and understand its implications for public health.
As the outbreak continues, it is vital for healthcare systems to adapt and respond proactively. This includes educating healthcare providers about the potential for drug-resistant monkeypox and the importance of reporting cases. The CDC has urged healthcare providers to maintain vigilance in their assessments and consider the possibility of tecovirimat resistance when treating mpox patients.
The Role of CDC in Monitoring Mpox Trends
The Centers for Disease Control and Prevention (CDC) play a crucial role in monitoring mpox trends and providing updates on the disease’s status. Their ongoing surveillance efforts have allowed for the early detection of tecovirimat-resistant variants, which is critical for managing the outbreak effectively. The CDC’s proactive approach includes disseminating information to healthcare providers about the potential risks associated with drug-resistant mpox and the need for alternative treatment strategies.
In light of the evolving nature of the monkeypox virus, the CDC continues to call for collaboration among public health labs and healthcare providers to ensure comprehensive tracking of mpox cases. This systematic monitoring will not only help in understanding the dynamics of the current outbreak but also inform future public health interventions aimed at controlling mpox transmission and safeguarding public health.
Clinical Trials: The Future of Mpox Treatment
Clinical trials are essential in advancing our understanding of mpox treatment options, particularly in the face of emerging tecovirimat resistance. The STOMP study, among others, is designed to evaluate the safety and efficacy of tecovirimat while exploring alternative therapeutic approaches. Participation in these trials is encouraged, as they provide valuable data that could lead to improved management strategies for mpox and contribute to the development of new treatments.
As researchers analyze the outcomes of these trials, the findings will be instrumental in shaping future treatment guidelines for mpox. By focusing on the nuances of the disease and the population’s response to different therapeutic agents, healthcare providers can better tailor their approaches to meet the needs of patients, particularly those who may be at risk of severe disease manifestations.
The Importance of Genomic Surveillance in Mpox
Genomic surveillance has emerged as a critical tool in the monitoring of mpox, especially with the identification of drug-resistant variants. By sequencing mpox case samples, public health labs can track the mutations associated with tecovirimat resistance and assess their spread across different regions. This information is invaluable for public health officials in making informed decisions about treatment protocols and outbreak management.
The experience gained from genomic surveillance during the mpox outbreak highlights the necessity of investing in advanced laboratory capabilities and infrastructure. As health authorities refine their surveillance strategies, continuous tracking of the monkeypox virus will be crucial in detecting similar events in the future, ensuring that public health measures can adapt to the evolving landscape of infectious diseases.
Addressing Public Concerns About Tecovirimat Use
Public health messaging regarding tecovirimat use must address the concerns surrounding its investigational status and potential for resistance. Clear communication about the current state of mpox treatment options, including the limitations of tecovirimat, is essential in maintaining public trust and encouraging adherence to health guidelines. As the CDC continues to provide updates on mpox, transparency about the risks and benefits of tecovirimat will help patients make informed decisions about their care.
Educational campaigns that target both healthcare providers and the public can help demystify the complexities of mpox treatment. By fostering an understanding of the ongoing research and the importance of clinical trials, health authorities can empower individuals to participate in studies that contribute to the development of effective therapies and ultimately improve health outcomes for those affected by mpox.
Collaboration in Mpox Research and Treatment Development
Collaboration among researchers, healthcare providers, and public health agencies is vital for the effective management of mpox. The emergence of tecovirimat-resistant variants demonstrates the need for a coordinated approach to research and treatment development. By pooling resources and expertise, stakeholders can more effectively address the challenges posed by the monkeypox virus and enhance the overall response to outbreaks.
Joint efforts in clinical research, data sharing, and public health initiatives can accelerate the development of alternative therapies and improve the understanding of mpox transmission dynamics. This collaborative framework will not only enhance the immediate response to the current outbreak but also build resilience against future infectious disease threats.
Monitoring the Impact of Travel on Mpox Spread
The impact of travel on the spread of mpox cannot be overstated, especially in light of recent cases linked to individuals traveling to areas with known tecovirimat-resistant strains. As public health officials trace the origins of these infections, it becomes evident that travel history plays a crucial role in understanding the transmission dynamics of mpox. Enhanced screening and monitoring at travel hubs may be necessary to prevent further outbreaks.
Health authorities must develop comprehensive guidelines for travelers, particularly those visiting regions with reported cases of mpox. By educating the public on the risks associated with travel and providing clear recommendations, officials can help mitigate the potential for further spread of the disease and protect vulnerable populations.
Frequently Asked Questions
What is tecovirimat resistance in mpox treatment?
Tecovirimat resistance in mpox treatment refers to the emergence of monkeypox virus strains that are no longer effectively treated by tecovirimat, the primary antiviral drug used against mpox. Recent studies have identified cases of tecovirimat-resistant MPXV in the United States, raising concerns about the effectiveness of current treatment options.
How is tecovirimat used in the context of the monkeypox outbreak 2024?
During the ongoing monkeypox outbreak in 2024, tecovirimat has been widely utilized as a treatment option for mpox. Although not FDA-approved specifically for mpox, it is available through compassionate use. The CDC emphasizes that while tecovirimat is considered safe, its effectiveness is still being evaluated, particularly in the context of emerging tecovirimat-resistant strains.
What are the implications of drug-resistant monkeypox on public health?
The emergence of drug-resistant monkeypox, particularly strains resistant to tecovirimat, poses significant public health challenges. It limits treatment options for patients, especially those who are severely immunocompromised. This situation highlights the urgent need for alternative mpox treatment options and ongoing genomic surveillance to monitor the spread of resistant variants.
What treatment options are available for mpox aside from tecovirimat?
Currently, tecovirimat is the primary treatment for mpox, but the emergence of tecovirimat-resistant strains underscores the necessity for additional mpox treatment options. Research is ongoing to identify and develop alternative therapeutics, and healthcare providers are encouraged to inform patients about clinical trials that may offer new treatment avenues.
What is the CDC’s stance on tecovirimat for mpox treatment?
The CDC acknowledges tecovirimat as a potential treatment for mpox but stresses that its use is still investigational. They have expressed concerns regarding the development of tecovirimat-resistant strains and urge healthcare providers to consider enrolling patients in clinical trials like STOMP to further understand the drug’s efficacy and safety in treating mpox.
How can individuals contribute to the research on tecovirimat and mpox?
Individuals can contribute to research on tecovirimat and mpox by participating in clinical trials, such as the NIH STOMP study. These trials are essential for evaluating the safety and effectiveness of tecovirimat in various patient populations, including those without severe disease. Participation helps advance knowledge about the treatment options available for mpox.
What mutations are associated with tecovirimat-resistant mpox cases?
Recent reports have identified a unique combination of F13 mutations linked to tecovirimat resistance in mpox cases. These mutations have been observed in 18 patients across several states, indicating a potential spread of drug-resistant monkeypox variants. Continued genomic surveillance is crucial to tracking these mutations and their impact on mpox treatment.
What are the risks of tecovirimat-resistant mpox infections?
The risks of tecovirimat-resistant mpox infections include limited treatment options and potential adverse outcomes, particularly for individuals with compromised immune systems. As tecovirimat may not be effective against resistant strains, healthcare providers must seek alternative therapies and closely monitor at-risk populations to mitigate severe manifestations of the disease.
What steps are being taken to monitor tecovirimat-resistant monkeypox?
Public health laboratories are conducting genomic surveillance to monitor the emergence and spread of tecovirimat-resistant monkeypox virus variants. This ongoing tracking is crucial for detecting similar events and ensuring that health authorities can respond promptly to protect public health.
| Key Points | Details |
|---|---|
| Tecovirimat Resistance | A cluster of mpox cases with a tecovirimat-resistant monkeypox virus has been identified in the U.S. |
| Previous Treatment History | All individuals in the cluster had no prior history of tecovirimat treatment. |
| Current Use of Tecovirimat | Tecovirimat is used for mpox but is not FDA approved, available only through compassionate use. |
| Clinical Trials | Ongoing trials such as the STOMP study are essential to evaluate tecovirimat’s efficacy. |
| F13 Mutations | A unique combination of F13 mutations linked to drug resistance was found in 18 patients. |
| Need for Alternatives | There is a pressing need for alternative treatments for mpox beyond tecovirimat. |
Summary
Mpox resistant tecovirimat is a significant concern as a new cluster of mpox cases caused by a tecovirimat-resistant monkeypox virus has emerged in the United States. This situation underscores the urgent need for effective monitoring and alternative treatment options for mpox, especially as the current use of tecovirimat is still investigational. Health authorities emphasize the importance of ongoing clinical trials to understand the full scope of tecovirimat’s effectiveness and to prepare for potential drug-resistant strains of the virus.
Leave a Reply