JYNNEOS vaccination is a crucial development in the fight against monkeypox, particularly for individuals with no prior exposure to smallpox or vaccinia virus. Recent studies have demonstrated a robust neutralizing antibody response following JYNNEOS vaccination, highlighting its effectiveness in generating immunity. Through the plaque reduction neutralization test (PRNT), researchers have assessed the durability of this response over a 12-month period, revealing promising results in protecting against MPXV. The study’s findings emphasize the importance of JYNNEOS as a modern smallpox vaccine, especially during outbreaks of related viruses. As public health efforts continue to evolve, understanding the implications of JYNNEOS vaccination is essential for effective vaccination strategies against viral threats like monkeypox.
The JYNNEOS vaccine represents a significant advancement in immunization strategies against monkeypox, often referred to in discussions about MPXV vaccination and viral containment. This innovative smallpox vaccine has been the subject of extensive research, focusing on its ability to elicit a strong immune response through neutralizing antibodies. Recent analyses, including those utilizing plaque reduction neutralization tests, have provided valuable insights into the vaccine’s efficacy and longevity of protection. By examining serum samples from vaccinated individuals, researchers are better equipped to understand the dynamics of the vaccinia virus analysis in the context of modern public health. As concerns about emerging viral infections grow, alternative terms like MPXV immunization and smallpox vaccine effectiveness highlight the ongoing relevance of JYNNEOS in current vaccination discourse.
Understanding the Neutralizing Antibody Response to JYNNEOS Vaccination
The neutralizing antibody response is a critical factor in evaluating the efficacy of any vaccination, including the JYNNEOS vaccine against monkeypox virus (MPXV). Recent studies have shown that individuals who received the JYNNEOS vaccine displayed a robust and sustained neutralizing antibody response over a 12-month period. This response is particularly important for smallpox vaccine-naive individuals, as it provides insights into the vaccine’s ability to protect against potential outbreaks of MPXV. The longitudinal data derived from plaque reduction neutralization tests (PRNT) offer vital information about the immune system’s capability to recognize and fight off the virus following vaccination.
In the context of the JYNNEOS vaccination, the plaque reduction neutralization test (PRNT) serves as a standard assay to gauge the effectiveness of the vaccine. The results from PRNT 50 and PRNT 90, which measure 50% and 90% plaque reduction respectively, indicate the levels of neutralizing antibodies present in the serum of vaccinated individuals. By analyzing serum from donors who received two doses of the JYNNEOS vaccine, researchers can derive geometric mean titers that reflect the immune response over time. This data underscores the importance of monitoring the duration and strength of the antibody response, especially in populations at risk for MPXV infection.
The Role of MPXV Vaccination in Public Health
MPXV vaccination, particularly with the JYNNEOS vaccine, plays a crucial role in public health strategies aimed at controlling outbreaks of monkeypox. The vaccine not only helps in generating a neutralizing antibody response but also contributes to herd immunity within populations. By vaccinating those at risk, health authorities can reduce the transmission of MPXV, thereby protecting vulnerable individuals who may not have immunity. Given the emerging concerns regarding the spread of monkeypox, understanding the public health implications of MPXV vaccination is more relevant than ever.
Furthermore, studies analyzing the long-term effects of the JYNNEOS vaccine are essential for formulating effective vaccination campaigns. Insights from smallpox vaccine studies indicate that the immune response can vary significantly among individuals, influenced by factors such as prior exposure to vaccinia virus. Monitoring the neutralizing antibody levels through assays like PRNT is vital for assessing the longevity of the vaccine’s protective effect. As monkeypox cases continue to be reported, the ongoing evaluation of MPXV vaccination strategies will be key in mitigating the impact of this zoonotic disease.
Analysis of Smallpox Vaccine Studies for Future Vaccination Strategies
Smallpox vaccine studies provide invaluable data that can inform future vaccination strategies, especially in the context of emerging diseases like monkeypox. The methodologies used, such as the plaque reduction neutralization test (PRNT), allow researchers to evaluate the effectiveness of vaccines like JYNNEOS. By examining the neutralizing antibody responses from various studies, public health officials can better understand the dynamics of immune response and tailor vaccination programs accordingly. Such analyses are essential for developing guidelines on booster shots and optimizing vaccination schedules.
Moreover, the historical context of smallpox eradication offers lessons that can be applied to the current fight against monkeypox. For instance, understanding the impact of vaccinia virus on immune response can help researchers predict how similar viruses may behave. The neutralizing antibody response observed in individuals vaccinated with JYNNEOS can serve as a benchmark for future studies. By leveraging data from past smallpox vaccine studies, health authorities can enhance their preparedness for potential MPXV outbreaks, ensuring that vaccination efforts are both effective and efficient.
The Plaque Reduction Neutralization Test (PRNT) Explained
The plaque reduction neutralization test (PRNT) is a widely used laboratory assay that evaluates the ability of antibodies to neutralize a virus. In the context of JYNNEOS vaccination, PRNT helps determine the efficacy of the neutralizing antibody response generated in vaccinated individuals. The test involves incubating serum samples with the virus, followed by measuring the resultant plaques formed in cell cultures. This method allows researchers to quantify the levels of neutralizing antibodies, which are crucial for assessing vaccine effectiveness against MPXV.
PRNT can be categorized into various metrics, such as PRNT 50 and PRNT 90, which signify the percentage of plaque reduction achieved. These metrics provide critical insights into the immune response elicited by the JYNNEOS vaccine over time. By conducting PRNT on serum samples collected longitudinally, researchers can map the decay of neutralizing antibodies and identify when booster vaccinations may be necessary. This detailed analysis is crucial for maintaining optimal immunity in populations at risk for monkeypox infection.
The Importance of Neutralizing Antibody Levels in Vaccine Efficacy
Neutralizing antibody levels are a key indicator of vaccine efficacy, particularly for vaccines like JYNNEOS. A robust neutralizing antibody response is essential for preventing viral infections, including those caused by MPXV. Studies have shown that higher levels of neutralizing antibodies correspond with better protection against infection and disease severity. Monitoring these antibody levels provides valuable insights into the long-term effectiveness of the JYNNEOS vaccine and informs recommendations for booster doses.
In assessments of the JYNNEOS vaccine, researchers utilize assays such as PRNT to determine the geometric mean titers of neutralizing antibodies. These measurements help public health officials understand how long the immune response lasts and when additional vaccinations may be warranted. As monkeypox cases continue to emerge globally, the ability to track and analyze neutralizing antibody levels will be vital for ensuring community immunity and preventing outbreaks.
Longitudinal Studies on JYNNEOS Vaccination and Immune Response
Longitudinal studies on JYNNEOS vaccination are essential for understanding the duration and strength of the immune response following vaccination. These studies track participants over an extended period, assessing how neutralizing antibody levels change over time. Such data is crucial for determining the need for booster shots and evaluating the overall effectiveness of the vaccine in preventing MPXV infections. By following smallpox vaccine-naive individuals, researchers can gather insights into how the immune system reacts to the JYNNEOS vaccine over a year or more.
The findings from these longitudinal studies also help clarify the relationship between prior vaccinia virus exposure and the immune response to the JYNNEOS vaccine. For individuals with no previous smallpox vaccination, the immune response may differ significantly compared to those with a history of vaccination. Understanding these dynamics is vital for tailoring public health strategies and ensuring that vaccination campaigns are equipped to provide adequate protection for all individuals, particularly in the context of emerging infectious diseases like monkeypox.
Evaluating the Impact of Vaccinia Virus on Immune Response
The vaccinia virus, a live virus used in smallpox vaccination, plays a significant role in shaping the immune response to subsequent vaccinations such as JYNNEOS. Individuals with prior exposure to vaccinia often exhibit enhanced neutralizing antibody responses due to the pre-existing immunity. This phenomenon underscores the importance of evaluating individual vaccination histories when assessing the effectiveness of new vaccines. By understanding how previous vaccinations influence immune responses, researchers can better predict the outcomes of JYNNEOS vaccination in diverse populations.
Furthermore, detailed analysis of the vaccinia virus’s impact on immune response can inform the development of future vaccines. By studying how different strains of vaccinia influence antibody production, scientists can optimize vaccines to elicit stronger and more durable immune responses. This knowledge is particularly relevant in the context of monkeypox, where effective vaccination strategies are crucial for preventing outbreaks and protecting public health.
Future Directions in MPXV Vaccination Research
As research continues to evolve, future directions in MPXV vaccination will focus on optimizing the JYNNEOS vaccine and enhancing our understanding of the immune response. Researchers aim to identify correlates of protection that can predict vaccine efficacy based on neutralizing antibody levels. This information will be vital for guiding vaccination strategies, particularly in at-risk populations. Additionally, ongoing studies will investigate the potential need for booster vaccinations and the timing of such interventions to maintain adequate immunity against MPXV.
Moreover, advancements in technology and assay methodologies will enhance our ability to monitor the immune response to MPXV vaccinations. Emerging techniques may provide more sensitive and specific measures of neutralizing antibodies, allowing for better tracking of vaccine efficacy over time. As monkeypox continues to pose a threat, the integration of innovative research approaches will be critical in developing effective vaccination strategies and ensuring public health safety.
Frequently Asked Questions
What is the neutralizing antibody response following JYNNEOS vaccination?
The neutralizing antibody response following JYNNEOS vaccination is a crucial indicator of vaccine efficacy, particularly against MPXV (monkeypox virus). Studies have shown that this response can persist for up to 12 months after receiving two doses of the JYNNEOS vaccine, which are administered approximately 28 days apart. The response is typically assessed using the plaque reduction neutralization test (PRNT), which measures the ability of antibodies to neutralize the virus.
How does JYNNEOS vaccination compare to previous smallpox vaccines in terms of efficacy?
JYNNEOS vaccination has been shown to provide an effective immune response, particularly in smallpox vaccine-naive individuals. In studies involving neutralizing antibody responses to MPXV, JYNNEOS demonstrated a significant increase in geometric mean titers when analyzed through plaque reduction neutralization tests (PRNT). While traditional smallpox vaccines also elicit a strong response, JYNNEOS is preferred for its safety profile, especially in immunocompromised individuals.
What role does the plaque reduction neutralization test (PRNT) play in assessing JYNNEOS vaccination?
The plaque reduction neutralization test (PRNT) is instrumental in evaluating the effectiveness of the JYNNEOS vaccination. This assay measures the neutralizing capacity of antibodies in serum samples obtained from vaccinated individuals. For JYNNEOS, PRNT results are categorized into PRNT 50 and PRNT 90, indicating the percentage of plaque reduction at different thresholds, which helps in determining the robustness of the immune response against MPXV and the vaccinia virus.
Can prior smallpox vaccination affect the response to JYNNEOS vaccination?
Yes, prior smallpox vaccination can influence the neutralizing antibody response to JYNNEOS vaccination. Individuals with previous vaccinia exposure may show different antibody responses compared to those who are smallpox vaccine-naive. In studies, data from participants with a history of smallpox vaccination often present distinct neutralizing antibody levels when assessed through plaque reduction neutralization tests (PRNT), indicating a potential enhancement in the immune response.
How long does the immune response last after JYNNEOS vaccination?
Research indicates that the immune response, specifically the neutralizing antibody response, can last for up to 12 months following JYNNEOS vaccination. This duration is assessed by monitoring geometric mean titers through plaque reduction neutralization tests (PRNT), ensuring that the vaccine provides sustained protection against MPXV.
| Key Points |
|---|
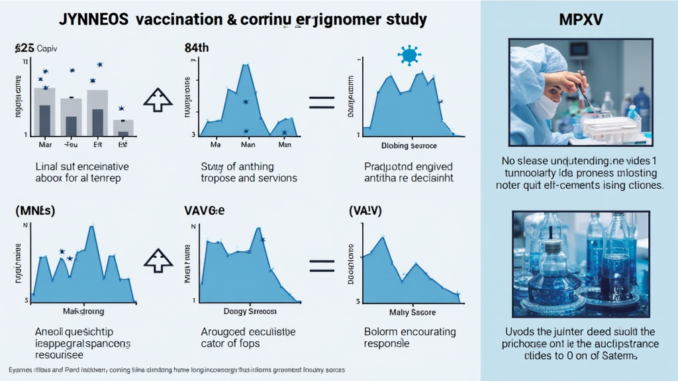
| JYNNEOS vaccination leads to a longitudinal neutralizing antibody response against monkeypox virus (MPXV) for up to 12 months. |
| The study involved smallpox vaccine-naive individuals receiving 2 doses of JYNNEOS approximately 28 days apart. |
| Plaque reduction neutralization tests (PRNT) were used to analyze serum samples from the vaccinated donors. |
| Results included PRNT 50 and PRNT 90 for both MPXV and vaccinia virus (VACV). |
| Data showed geometric mean titers with limits of detection highlighted in the results. |
| The analysis included data from individuals with prior smallpox vaccination as well as those without. |
Summary
JYNNEOS vaccination is shown to provide a significant neutralizing antibody response against MPXV, lasting up to 12 months post-vaccination. This response is crucial for individuals who are naive to smallpox vaccination, as it offers protection against monkeypox. The study utilized plaque reduction neutralization tests to assess the efficacy of the vaccine, demonstrating robust immune responses that are essential for public health, especially in the context of rising monkeypox cases.
Leave a Reply